
Dr. Franziska Hentzschel
franziska.hentzschel
(at)med.uni-heidelberg.de
Phone: +49 6221 56–7877
Malaria Research
Projects and research interest
In humans, Plasmodium parasites cause malaria, a deadly disease killing hundreds of thousands of people each year. The sexual replication of Plasmodium, however, occurs in the mosquito, starting with the formation of male and female gametes (gametogenesis). Male gametogenesis is particularly fascinating, as within only ten minutes, eight male gametes form and emerge from one single parent cell. In this time the parent cell replicates its DNA three times to provide eight genomes, but its nucleus only divides when the eight gametes emerge from the cell. It is thus a challenge for the parasite to make sure that each gamete takes up a complete genome from the single nucleus. Failure to do so results in male gametes with too little DNA, and while they can still fertilise female gametes and form zygotes, these will then stop developing at the oocyst stage in the mosquito. A functional and complete male genome is thus essential for the parasite to transmit through the mosquito.
At the Hentzschel lab, we investigate the formation and contribution of the male genome to the sexual replication of Plasmodium. How does the male gametocyte ensure that each gamete takes up a complete genome? What does the male gamete contribute to the female gamete during fertilisation to initiate development of a motile zygote? How is replication organised in the oocyst and why does the oocyst require a diploid genome for this? We address these research questions using a combination of reverse genetics, live cell and fixed imaging techniques, and interactome studies to reveal the molecular basis for these phenotypes. In addition, we develop novel genetic tools to enable investigation of gene function in the diploid oocyst stages.
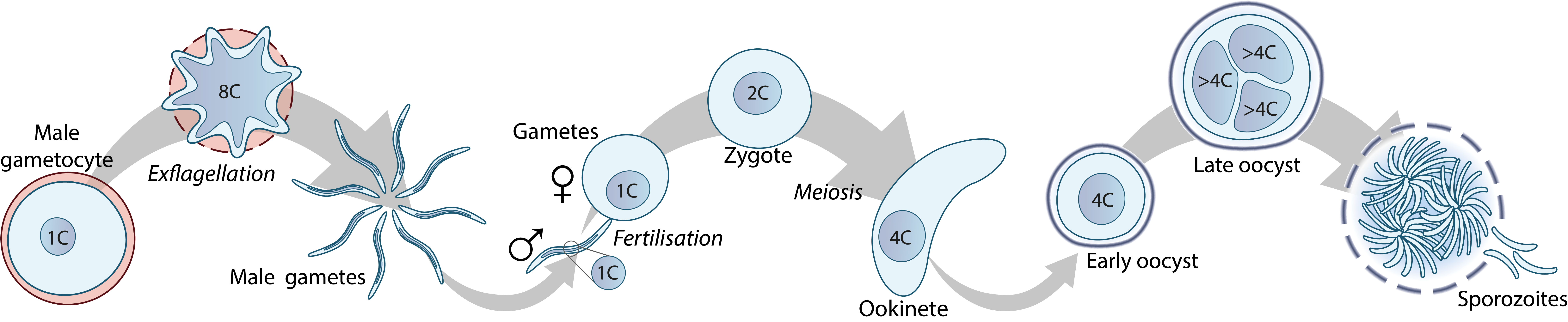
Figure 1. Plasmodium parasite development in the mosquito, from gametocytes to oocysts. C denotes the genome content.
1 | Deciphering DNA segregation during Plasmodium male gametogenesis
During the rapid formation of male gametes, the cell must ensure that each of the eight budding gametes takes up one genome from the octoploid nucleus. We have discovered that Plasmodium utilise a atypical Actin-related protein 2/3 complex (Arp2/3) to mediate this DNA segregation. We are now investigating its mechanistic function and how this protein complex is assembled and activated. We also investigate the function of further interaction partners to gain a deeper understanding of the molecular biology of male gamete formation.
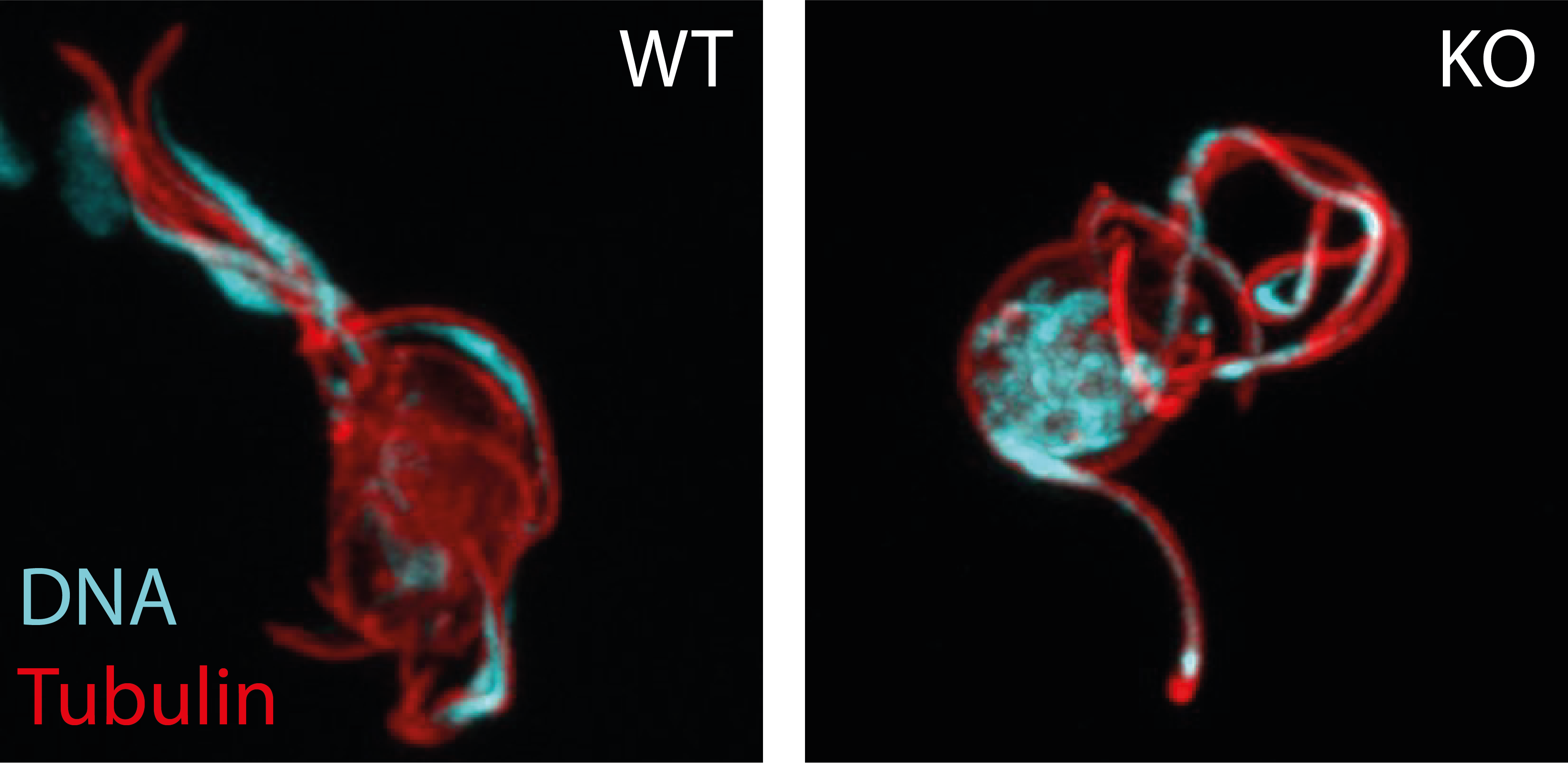
Figure 2. Male gametes emerging from the parent cell. DNA is stained in cyan, microtubule are stained in red. While in the wildtype (WT), gametes take up the DNA into the emerging gametes, parasites lacking the protein complex (KO) fail to do so.
2 | Developing novel CRISPR tools to dissect the mosquito stages of Plasmodium
The molecular and genetic basis of the development of Plasmodium in mosquitoes is little understood. One reason for this is that genetic modifications can only be done in blood stages, and if a gene deletion arrests parasite development there, the function of this gene cannot be investigated in subsequent oocyst stages. Another reason is that oocysts are diploid, carrying both the maternal and the paternal genome, which further complicates reverse genetics. We are working on engineering CRISPR-based gene targeting strategies that make use of the sexual replication of Plasmodium to silence gene expression only in the diploid oocyst.
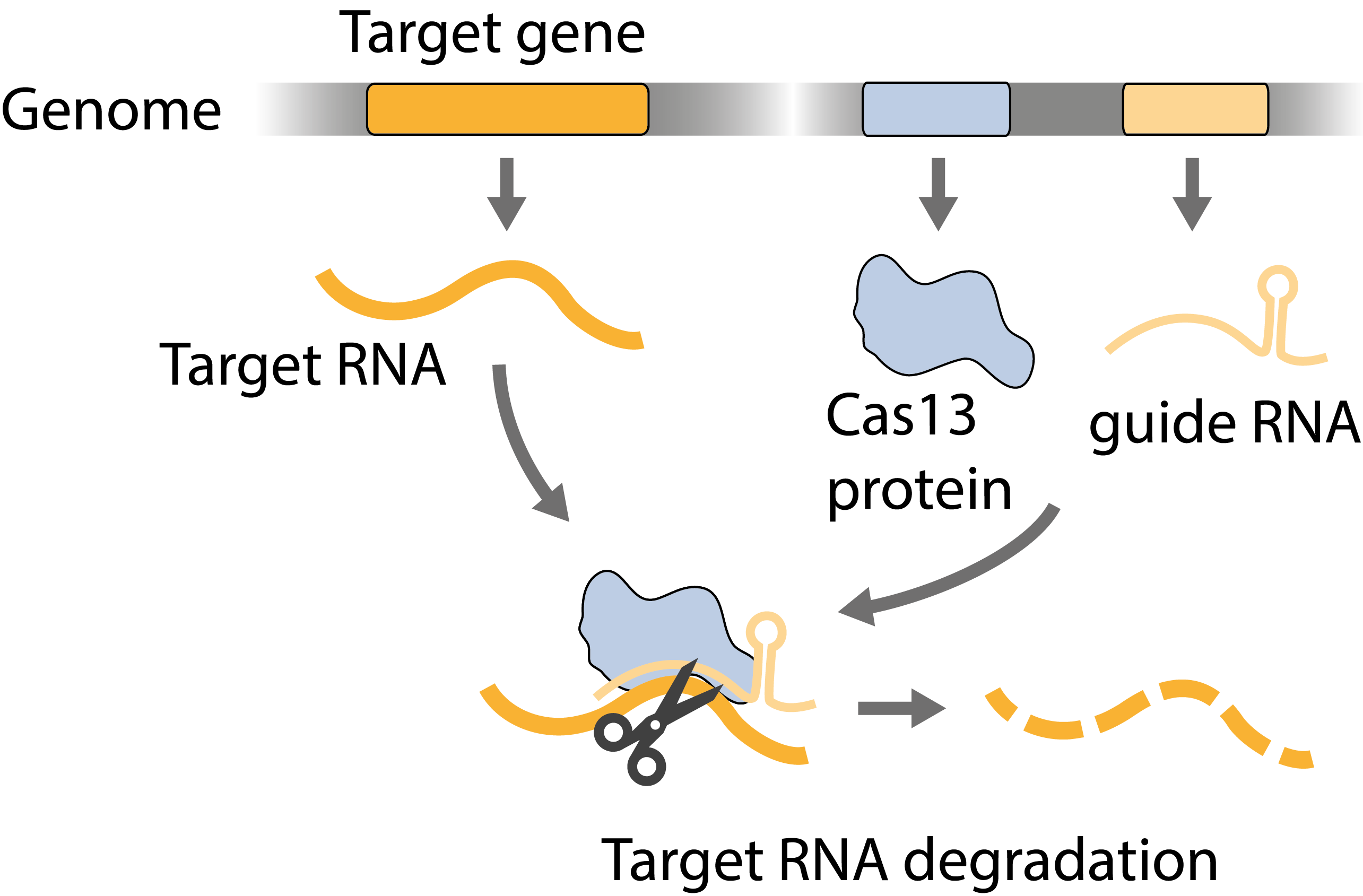
Figure 3. Scheme of post-transcriptional gene silencing.
3 | Investigating the early replication events in the oocyst
The oocyst is a highly unusual, underexplored life cycle stage of Plasmodium. Not only is it the only stage at which the parasite grows extracellularly, it also employs an unusual mode of replication where DNA replication is not always followed by nuclear division, and the oocyst thus contains multiple nuclei with several genomes each. Curiously, we found that if oocysts are aneuploid, i.e. missing a subset of the paternal genome, oocysts arrest early in development. Using live-cell imaging approaches, single-cell transcriptomics and reverse genetics, we aim to spatiotemporally characterize early oocyst development and to understand how the paternal genome is important for this development.
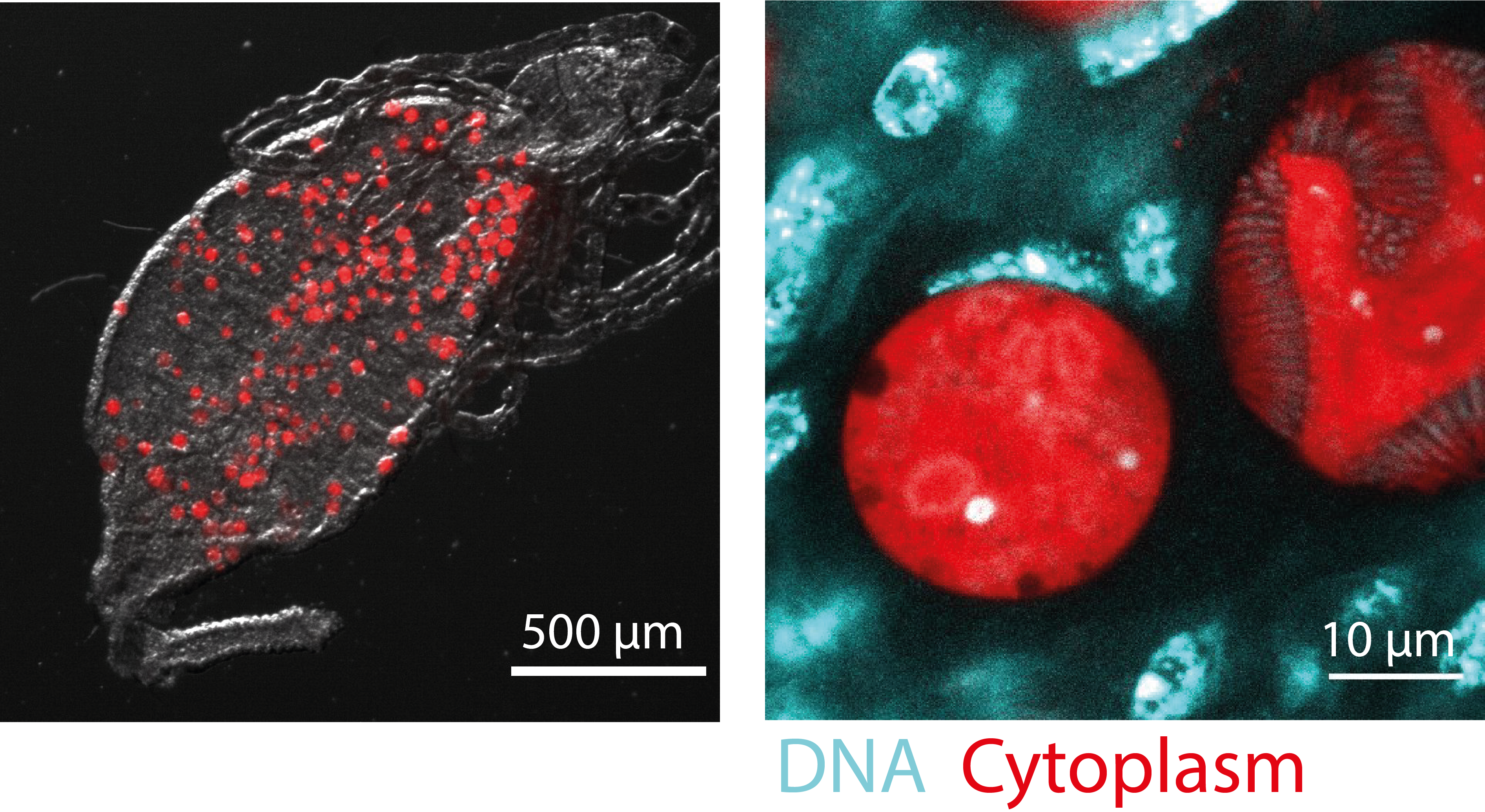
Figure 4. Oocyst development. Left: Oocysts growing on a mosquito midgut. Right: Close-up on two oocysts (red), one of which is already producing daughter cells (sporozoites). DNA of oocysts and surrounding mosquito cells is stained in cyan.
Research articles
- Hentzschel, F., Jewanski, D.*, Sokolowski, Y.*, Agarwal, P., Kraeft, A., Hildenbrand, K., Dorner, L. P., Singer, M., Frischknecht, F., & Marti, M. (2023). A non-canonical Arp2/3 complex is essential for Plasmodium DNA segregation and transmission of malaria. BioRxiv, 2023.10.25.563799. DOI: 10.1101/2023.10.25.563799 (accepted at Nat Micro)
- Hentzschel, F.*, Binder, A. M.*, Dorner, L. P., Herzel, L., Nuglish, F., Sema, M., Aguirre-Botero, M. C., Cyrklaff, M., Funaya, C., & Frischknecht, F. (2023). Microtubule inner proteins of Plasmodium are essential for transmission of malaria parasites. BioRxiv, 2023.10.19.562943. DOI: 10.1101/2023.10.19.562943 (accepted at PNAS)
- Hentzschel, F., Gibbins, M. P., Attipa, C., Beraldi, D., Moxon, C. A., Otto, T. D., & Marti, M. (2022). Host cell maturation modulates parasite invasion and sexual differentiation in Plasmodium berghei. Sci Adv, 8(17), 7348. DOI: 10.1126/SCIADV.ABM7348
- Hentzschel, F., Mitesser, V., Fraschka, S., Krzikalla, D., Carrillo, E., Berkhout B., Bártfai R., Mueller, A.-K., & Grimm, D. (2020) Gene knockdown in malaria parasites via non-canonical RNAi. NAR, 48(1), e2. DOI: 10.1093/nar/gkz927
- Hentzschel, F.*, Hammerschmidt-Kamper, C.*, Börner, K.*, Heiss, K.*, (…), Mueller, A‑K. M., Grimm, D. (2014) AAV8-mediated in vivo overexpression of miR-155 enhances the protective capacity of genetically-attenuated malarial parasites. Mol. Ther., 22(12), 2130–2141. DOI: 10.1038/mt.2014.172
Reviews
- Hentzschel, F., & Frischknecht, F. (2022). Still enigmatic: Plasmodium oocysts 125 years after their discovery. Trends in Parasitology, 38(8), 610–613. DOI: 10.1016/J.PT.2022.05.013 (Review)
- Venugopal, K., Hentzschel, F., Valkiūnas, G., Marti, M. (2020) Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat Rev Micro, 18(3), 177–189. DOI: 10.1038/s41579-019‑0306‑2 (Review)
- Ngotho P., Soares AB., Hentzschel F., Achcar F., Bertuccini L., Marti M. (2019) Revisiting gametocyte biology in malaria parasites. FEMS Microbiol Rev., 43(4), 401–414. DOI: 10.1093/femsre/fuz010 (Review)
