
Nuclear division mechanisms in malaria-causing parasite
Projects
Despite being key to rapid proliferation of malaria-causing parasites in the human blood the mechanisms of cell division are still poorly understood. Our vision is to generate a cell biological and genetic framework, which details critical events during this atypical mode of nuclear division. The study of this highly dynamic and small scale process requires exquisite temporal and spatial resolution. Hence, we are using a combination advanced imaging techniques such as live cell, super-resolution and correlative light and electron microscopy to investigate key structural elements of the division machinery. Thereby we hope to uncover new targets within this essential pathway of the parasite’s life cycle.
1 | Organization and composition of the atypical plasmodial centrosomes (Caroline Sophie Simon)
Centrosomes are microtubule organizing centers and master regulators of mitosis. Studying their functionality and composition is critical to understand cell division. The plasmodial centrosomes, however, display a very distinct morphology when compared to any model organism. Based on the poor conservation of canonical centrosome proteins we speculate that divergent factors might govern mitosis in the parasite. We aim to build the first detailed working model for centrosome organization using advanced imaging methods such as STED nanoscopy. To uncover the centrosomal proteome we use in-vivo proximity labeling and mass spectrometry.
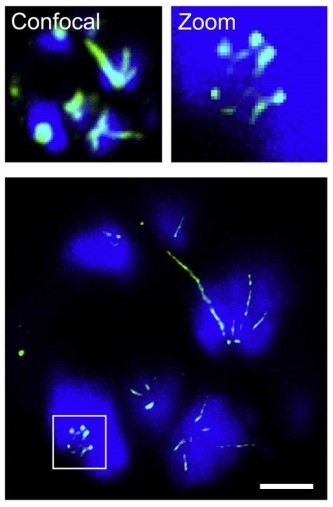
Figure 1 | STED nanoscopy of dividing parasite. Image acquired by RescueSTED showing late stage P. falciparum multinucleated parasite, which has infected a human red blood cell. DNA (blue) is stained by Hoechst and microtubules (green) are labeled with anti-Tubulin antibody. Confocal images and zoomed in region are shown on top. Scale bar, 1µm.
2 | Centrin function in malaria parasite division (Yannik Voß)
Centrins are conserved structural proteins involved in centrosome duplication in mammals. P. falciparum possesses four homologues of which two are specific for the phylum of this parasite. In model organisms centrins have been described as calcium-binding contractile elements with the potential to polymerize. We aim to investigate the function of those, likely essential, proteins by inducible knock-out strategies. We complement our in vivo studies with in-vitro biochemical assays using recombinant centrins to test for polymerization, post-translational modification, and interaction partners.
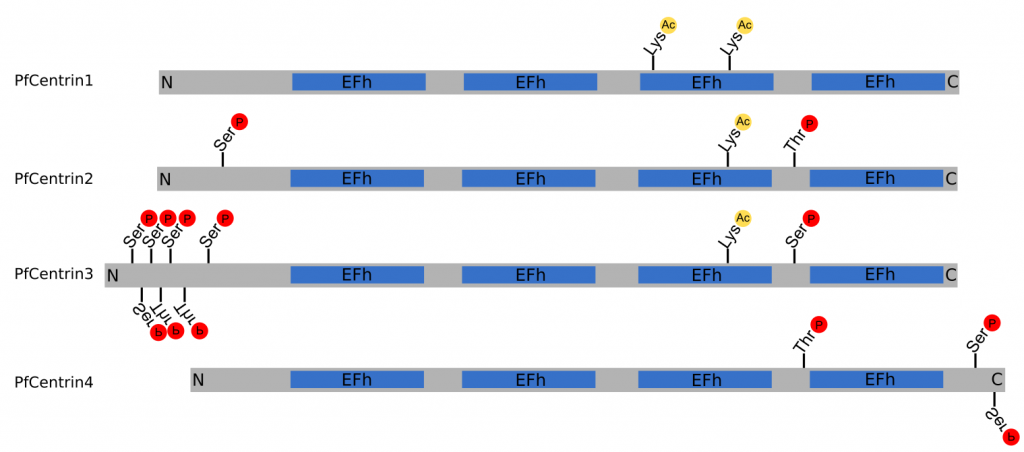
Figure 2 | Structure of PfCentrin1‑4. Schematic indicating the relative size of PfCentrin1‑4, as well as the position of the EF-hand domains (EFh), phosphorylation sites ℗ and acetylation sites (Ac) to scale. N‑terminus (N), C‑terminus © (Cobbold et al., 2016; Ganter et al., 2017; Treeck et al., 2011).
Selected Publications
Complete publication list (PubMed)
Mehnert, A.K., Simon C.S., Guizetti, J. (2019). Immunofluorescence staining protocol for STED nanoscopy of Plasmodium-infected red blood cells. Mol Biochem Parasitol. 229, 47–52.
Müller, L. S. M., Cosentino, R. O., Förstner, K. U., Guizetti, J., Wedel, C., Kaplan, N., … Siegel, T. N. (2018). Genome organization and DNA accessibility control antigenic variation in trypanosomes. Nature 563(7729), 121–125
Zanghì, G., Vembar, S. S., Baumgarten, S., Ding, S., Guizetti, J., Bryant, J. M., … Scherf, A. (2018). A Specific PfEMP1 Is Expressed in P. falciparum Sporozoites and Plays a Role in Hepatocyte Infection. Cell Reports 22(11), 2809–2817.
Bryant J.M., Regnault C., Scheidig-Benatar C., Baumgarten S., Guizetti J.*, Scherf A. (2017). CRISPR/Cas9 Genome Editing Reveals That the Intron Is Not Essential for var2csa Gene Activation or Silencing in Plasmodium falciparum. MBio 8(4). pii: e00729-17.
Guizetti, J.*, Barcons-Simon, A., Scherf, A. (2016). Trans-acting GC-rich non-coding RNA at var expression site modulates gene counting in malaria parasite. Nucleic Acids Res 44, 9710–9718.
Zhang, Q., Siegel, T. N., Martins, R. M., Wang, F., Cao, J., Gao, Q., Cheng, X., Jiang, L., Hon, C. C., Scheidig-Benatar, C., Sakamoto, H., Turner, L., Jensen, A. T., Claes, A., Guizetti, J., Malmquist, N. A., and Scherf, A. (2014). Exonuclease-mediated degradation of nascent RNA silences genes linked to severe malaria. Nature 513, 431–435.
Guizetti, J., Martins, R. M., Guadagnini, S., Claes, A., and Scherf, A. (2013). Nuclear Pores and Perinuclear Expression Sites of var and Ribosomal DNA Genes Correspond to Physically Distinct Regions in Plasmodium falciparum. Eukaryot Cell 12, 697–702.
Guizetti, J., Schermelleh, L., Mantler, J., Maar, S., Poser, I., Leonhardt, H., Muller-Reichert, T., and Gerlich, D. W. (2011). Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331, 1616–1620.
Guizetti, J., Mantler, J., Muller-Reichert, T., and Gerlich, D. W. (2010). Correlative time-lapse imaging and electron microscopy to study abscission in HeLa cells. Methods Cell Biol 96, 591–601.
Lacroix, B., van Dijk, J., Gold, N. D., Guizetti, J., Aldrian-Herrada, G., Rogowski, K., Gerlich, D. W., and Janke, C. (2010). Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol 189, 945–954.
Lekomtsev, S., Guizetti, J., Pozniakovsky, A., Gerlich, D. W., and Petronczki, M. (2010). Evidence that the tumor-suppressor protein BRCA2 does not regulate cytokinesis in human cells. J Cell Sci 123, 1395–1400.
Steigemann, P., Wurzenberger, C., Schmitz, M. H., Held, M., Guizetti, J., Maar, S., and Gerlich, D. W. (2009). Aurora B‑mediated abscission checkpoint protects against tetraploidization. Cell 136, 473–484.
Reviews
Guizetti, J.*, and Scherf, A. (2013). Silence, activate, poise and switch! Mechanisms of antigenic variation in Plasmodium falciparum. Cell Microbiol 15, 718–726.
Guizetti, J., and Gerlich, D. W. (2012). ESCRT-III polymers in membrane neck constriction. Trends Cell Biol 22, 133–140.
Guizetti, J., and Gerlich, D. W. (2010). Cytokinetic abscission in animal cells. Semin Cell Dev Biol 21, 909–916.
