
Prof. Dr. Friedrich Frischknecht
freddy.frischknecht@med.uni-heidelberg.de
Phone: ++49-(0)6221–56-6537
From Plasmodium sporozoite biology to interventions against malaria
Projects
We study malaria parasites because they are both important human pathogens and divergent eukaryotic cells featuring a unique biology. Our lab is mainly interested in understanding the role of diverse proteins in the motility of malaria parasite, primarily Plasmodium sporozoites the forms transmitted by the mosquito. However, we also study other forms that rely on microtubules or actin to move within and across different tissues. We also investigate new ways to diagnose and potentially control malaria.
1 | Identification of new Plasmodium biology in the mosquito
To infect the mosquito and be efficiently transmitted, the malaria parasite needs to move. In order to understand how parasites move we first need to know which proteins are essential for motility. To identify new candidate proteins that are secreted by the parasites at different live cycle stages (gametocytes, ookinetes, sporozoites) we use proteomic approaches. This has identified a large number of novel proteins that we are now characterizing by the generation of gene deletion mutants and parasite lines expressing fluorescently tagged proteins. We also express some of them in vitro for biochemical and structural analysis.
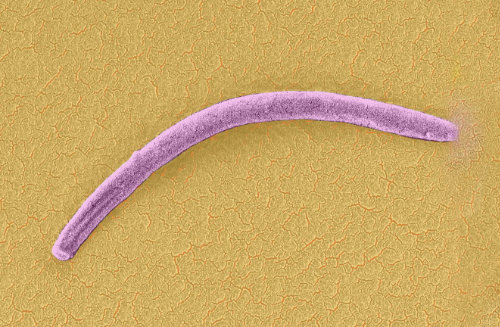
Figure 1| Plasmodium sporozoite as seen by scanning electron microscopy (false color added). Photo by Leandro Lemgruber.
2 | Understanding the divergent function of conserved proteins
Several proteins are known key players in sporozoite motility, including trans-membrane adhesion proteins at the plasma membrane and proteins of the actin cytoskeleton. The adhesion proteins contain conserved domains found across most eukaryotes. However there are interesting differences in their ligand specificity, which we aim to understand by generating parasite lines expressing adhesion proteins that contain domains from different organisms. Also actin and actin binding proteins are very important for sporozoite motility. Again, the actin looks very similar to actin from yeast or humans, however, in Plasmodium it only makes short and highly dynamic filaments. We aim to identify which amino acids in actin are causing these fundamentally different characteristics. Also some very conserved actin binding proteins contain domains not known from other organisms or function in analogous, yet different ways and we address these by a mix of genetic, biochemical and cell biology approaches.
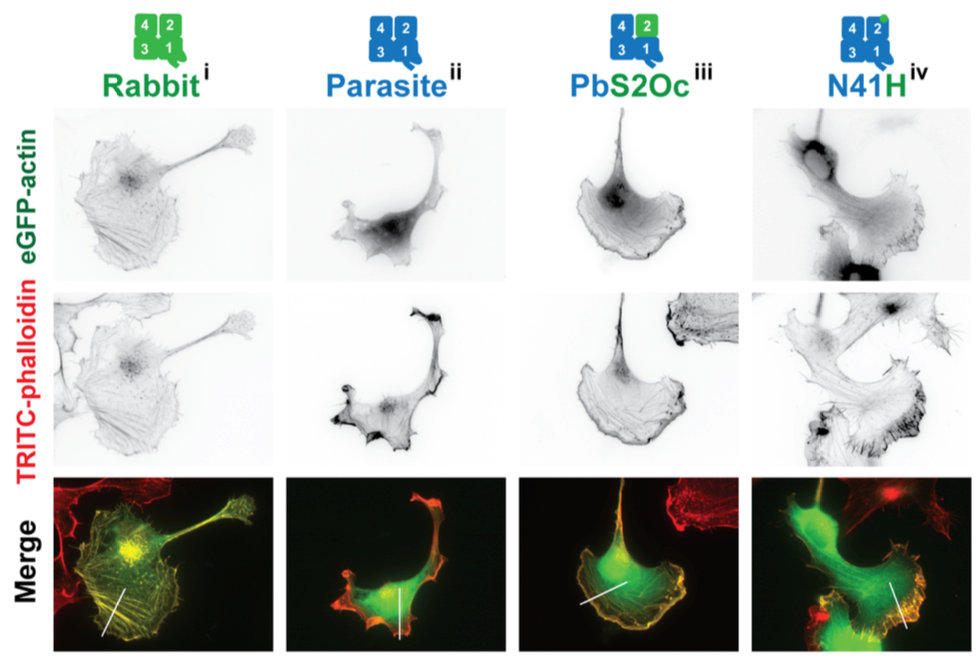
Figure 2 | Expression of actin from rabbit muscle or Plasmodium or chimeric proteins in mammalian cells shows their distinct localization properties. The single point mutant N41H can allow Plasmodium actin to localize like rabbit actin. From Douglas et al., Plos Biol., 2018.
3 | Generation of new types of attenuated parasites for experimental vaccinations
Parasite lines that arrest their growth in the liver have been used for experimental vaccinations as well as in several clinical trials. Through genetic modification such parasite lines can be generated yet sometimes the arrest is not 100% leading to infections and disease while sometimes the efficacy of the attenuated parasite to protect from disease is low. We work on new types of attenuated parasites that arrest their growth either in the liver or the blood stage and test them in mice with the ultimate goal of translating the results into clinical trials.
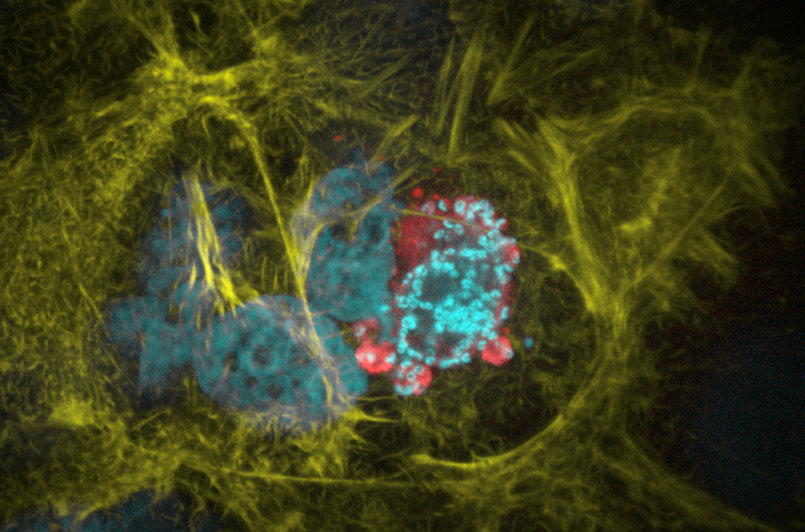
Figure 4 | Liver cell infected with a Plasmodium parasite. Cyan shows the live cell nuclei as well as the small nuclei of the parasite. In red the parasite cytoplasm and in yellow the host cell actin cytoskeleton are visualized. Photo by Mirko Singer.
4 | Development of new diagnostic tools
We recently started to collaborate with two companies, Intuity and Zendia to develop new ways of diagnosing malaria. While Intuity build a cheep microscope and an algorithm based on artificial intelligence to spot and count the parasites in a blood smear, Zendia aims at developing a rapid diagnostic test based on the fluorescent detection of parasites.
Complete Publication List (PubMed)
- Frischknecht, F., Moreau, V., Röttger, S., Gonfloni, S., Reckmann, I., Superti-Furga, G. and Way, M. (1999) Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling, Nature, 401:926–929.
- Amino, R., Thiberge, S., Martin, B., Celli, S., Shorte, S. L., Frischknecht, F. and Ménard R. (2006) Quantitative imaging of malaria parasite transmission to the mammalian host, Nat. Med. 12:220–224.
- Cyrklaff, M., Kudryashev, M., Leis, A., Leonard, K., Baumeister, W., Ménard, R., Meissner, M., and Frischknecht, F. (2007) Cryoelectron tomography reveals periodic luminal material in subpellicular microtubules from apicomplexan parasites, J. Exp. Med. 204:1281–1287.
- Münter, S., Sabass, B., Selhuber-Unkel, C., Kudryashev, M., Hegge, S., Spatz, J. P., Engel, U., Matuschewski, K., Schwarz, U. S. and Frischknecht, F. (2009) Plasmodium sporozoite motility is modulated by the turnover of discrete adhesion sites, Cell Host Microbe 6:551–562.
- Hegge, S., Münter, S., Steinbüchel, M., Heiss, K., Engel, U., Matuschewski, K. and Frischknecht, F. (2010), Multistep adhesion of Plasmodium sporozoites, FASEB J. 24:2222–2234.
- Hellmann, J. K., Münter, S., Kudryashev, M., Schulz, S., Heiss, K., Müller, A.-K. Matuschewski, K., Spatz, J. P., Schwarz, U. S. and Frischknecht, F. (2011) Environmental constraints guide migration of malaria parasites during transmission, PLoS Pathog, 7:e1002080.
- Kudryashev, M., Münter, S., Lemgruber, L., Montagna, G., Matuschewski, K., Stahlberg, H., Meissner, M., Cyrklaff, M. and Frischknecht, F. (2012) Structural basis for chirality and directed Plasmodium sporozoite migration, Cell. Microbiol. 14:1757–1768.
- Singer, M., Marshall, J., Heiss, K., Mair, G. R., Grimm, D., Mueller A. K. and Frischknecht, F. (2015) Zinc-finger nuclease-based double strand breaks attenuate malaria parasites and reveal rare micro-homology mediated end joining, Genome Biol. 16:249.
- Quadt, K. A., Streichfuss, M., Moreau, C. A. Spatz, J. P. and Frischknecht, F. (2016) Coupling of retrograde flow to force production during malaria parasite migration, ACS Nano 10:2091–2102.
- Kumar, H., Sattler, J. M., Singer M., Heiss, K., Reinig, M., Hammerschmidt-Kamper, C., Heussler, V. T., Mueller, A.K. and Frischknecht F. (2016) Protective efficacy and safety of liver stage attenuated malaria parasites, Sci. Rep. 6:26824.
- Kehrer, J., Frischknecht, F. and Mair G. R. (2016) Proteomic analysis of the Plasmodium berghei gametocyte egressome and vesicular bioID of osmiophilic body proteins identified MTRAP as an essential factor for parasite transmission, Mol. Cell. Proteom. 15:2852–2862.
- Bane, K., Lepper, S., Kehrer, J., Sattler, J. M., Singer, M., Reinig, M., Heiss, K., Baum, J., Mueller, A. K. and Frischknecht, F. (2016) The actin filament-binding protein coronin regulates motility in Plasmodium sporozoites, PLoS Pathog. 12:e1005710.
- Moreau, C., Bhargav, S. P., Kumar, H. Quadt, K., Piirainen, H., Strauss, L., Kehrer, J., Streichfuss, M., Spatz, J. P., Wade, R. C., Kursula, I. and Frischknecht, F. (2017) A unique profilin-actin interface is important for malaria parasite motility, PLoS Pathog. 13:e1006412.
- Klug, D. and Frischknecht, F. (2017) Motility precedes egress of malaria parasites from oocysts, eLife, e19157.
- Douglas, R., Nandekar, P., Aktories, J.-E., Kumar, H., Weber, R., Singer, M., Lepper, S., Sadiq, S. K., Wade, R. C. and Frischknecht, F. (2018) Inter-subunit interactions drive divergent dynamics in mammalian and Plasmodium actin filaments, PLoS Biol. 16:e2005345.
- Spreng, B., Fleckenstein, H., Kübler, P., Di Biagio, C., Benz, M., Patra, P., Schwarz, U. S., Cyrklaff, M. and Frischknecht, F. (2019) Microtubule number and length determine cellular shape and function in Plasmodium, EMBO J. 38:e100984.
- Klug D, Goellner S, Kehrer J, Sattler J, Strauss L, Singer M, Lu C, Springer TA, Frischknecht F. (2020) Evolutionarily distant I domains can functionally replace the essential ligand-binding domain of Plasmodium TRAP. Elife. 9:e57572.
- Ripp J, Kehrer J, Smyrnakou X, Tisch N, Tavares J, Amino R, Ruiz de Almodovar C, Frischknecht F. (2021) Malaria parasites differentially sense environmental elasticity during transmission. EMBO Mol Med. (4):e13933.
Review Articles:
- Frischknecht, F. and Fackler, O. T. (2016) Experimental systems for studying Plasmodium-HIV coinfections, FEBS Lett. 590:2000–2013. (review)
- Frischknecht, F. and Matuschewski, K. (2017) Plasmodium sporozoite biology, in D. Wirth and P. Alonso (eds), Malaria: Biology in the Era of Eradication, Cold Spring Harb. Perspect. Med. 7:a025478. (review)
- Singer, M. and Frischknecht, F. (2017) Time for genome editing: next-generation attenuated malaria parasites, Trends Parasitol. 33, 202–213, 2017. (review)
- Cyrklaff, M., Frischknecht, F. and Kudryashev, M. (2017) Functional insights into pathogen biology from 3D electron microscopy, FEMS Microbiol. Rev. 41:828–853. (review)
- De Niz M, Spadin F, Marti M, Stein JV, Frenz M, Frischknecht F. (2019) Toolbox for In Vivo Imaging of Host-Parasite Interactions at Multiple Scales. Trends Parasitol. 35:193–212.
- Harding CR, Frischknecht F. (2020) The Riveting Cellular Structures of Apicomplexan Parasites. Trends Parasitol. 36:979–991.
