
Understanding Mechanisms of Long-Term Viral Sequelae
Projects
Our research focuses on how viral infections alter host signalling networks and immune responses, driving sustained inflammation and post-viral pathology. We aim to uncover the molecular and cellular mechanisms that underlie post-viral syndromes, with a particular focus on long COVID.
Long COVID, or post-acute sequelae of SARS-CoV‑2 infection (PASC), has emerged as a major global health challenge, affecting millions of people long after the initial infection. Despite its prevalence, the biological causes of these persistent symptoms remain poorly understood, and no diagnostic markers or targeted therapies currently exist. Many patients experience fatigue, cognitive impairment and organ dysfunction months to years after infection, underscoring the urgent need for mechanistic insight and novel treatment strategies.
Dysregulated immune and inflammatory pathways contribute to long COVID, yet the host factors that transform an acute infection into a chronic, multi-organ inflammatory state remain elusive. To address this complexity, we take a multidisciplinary approach that bridges virology, immunology, and translational medicine. Our lab develops and applies innovative experimental platforms – including advanced imaging, multi-omics profiling, and RNA-based therapeutic delivery systems – to uncover disease mechanisms and evaluate potential interventions.
Defining the Molecular Drivers of Long-Term Pathology
Programmed cell death is critical for controlling infection and restoring tissue integrity, but when these processes are dysregulated, they can sustain persistent inflammation. Many viruses exploit cell death pathways to their advantage, shifting the balance from host defence toward viral persistence. We study how cell death and inflammatory pathways are rewired following infection and how this dysregulation drives the shift from an effective acute response to chronic disease.
During acute SARS-CoV‑2 infection, we have shown that the cell death protein caspase‑8 is diverted from its canonical apoptotic function to instead propagate inflammatory signalling. Caspase‑8 accumulates in the lung during infection and remains dysregulated for months after viral clearance (Figure 1). Our current work aims to uncover the molecular mechanisms underlying this sustained imbalance by identifying the signalling nodes that remain aberrantly activated and contribute to long-term disease. Using integrated transcriptomic, proteomic, and high-resolution imaging approaches in vivo, we map the networks that maintain chronic inflammation and investigate how they may be precisely modulated to restore immune balance and promote tissue repair.
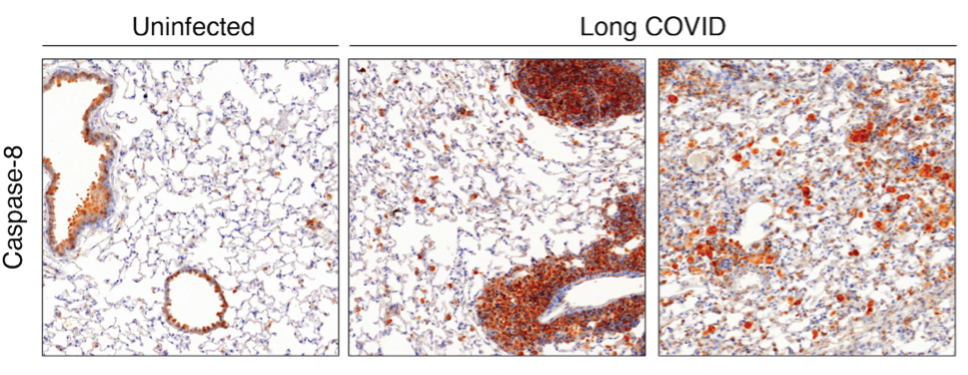
Figure 1: Immunohistochemical staining of lung sections highlighting caspase‑8 expression. Mice with prior SARS-CoV‑2 infection exhibit markedly increased caspase‑8 levels compared to naïve controls, and this elevated expression persists for months following viral clearance.
Linking Viral Infection to Neurodegenerative Disease
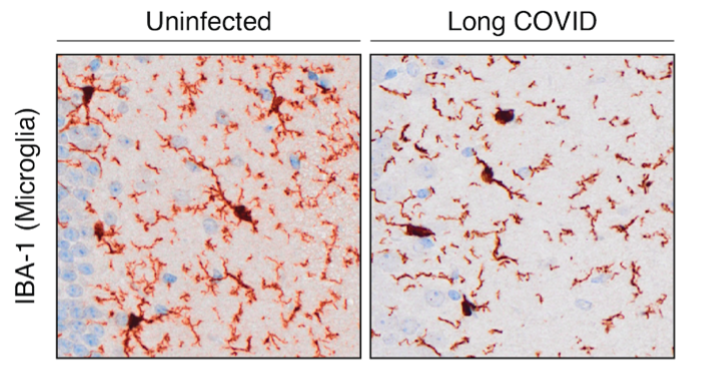
Epidemiological studies show that individuals recovering from COVID-19 have a higher risk of developing neurodegenerative diseases, including Alzheimer’s and Parkinson’s, but the biological mechanisms linking infection to neurological decline remain unclear. We investigate how prior viral infection alters neuronal and glial function, promotes inflammatory signalling, and affects brain health (Figure 2). Using behavioural testing platforms, transcriptomics, and single-cell approaches, we aim to uncover how persistent immune dysregulation contributes to long-term neurological changes.
Figure 2: Immunohistochemical staining of microglia. Long COVID leads to activation and changes in microglial morphology.
Translating Disease Mechanisms into Therapies
By integrating experimental preclinical data with clinical collaborations, we connect molecular signatures of immune dysregulation to patient outcomes, enabling the identification of biomarkers and therapeutic targets for post-viral diseases. Our work also explores host-directed and RNA-based strategies to precisely modulate key pathways involved in chronic inflammation, while maintaining essential immune functions. This includes both the repurposing of clinical-stage compounds and the development of lipid nanoparticle (LNP)-mediated RNA delivery systems for targeted therapeutic intervention.
Our Strategy and Objectives
Through this multidisciplinary research programme, the Bader Lab aims to unravel how viral infections disrupt immune homeostasis and to translate mechanistic discoveries into precision therapies (Figure 4). By linking fundamental biology with clinical application, we seek to prevent and reverse the long-term consequences of infection — ultimately improving outcomes for people affected by long COVID and related post-infectious disorders.
We are an ambitious, collaborative, and international team, and we welcome applications from motivated students and technical assistants who are excited to contribute to cutting-edge research at the intersection of immunology, virology, and translational medicine.
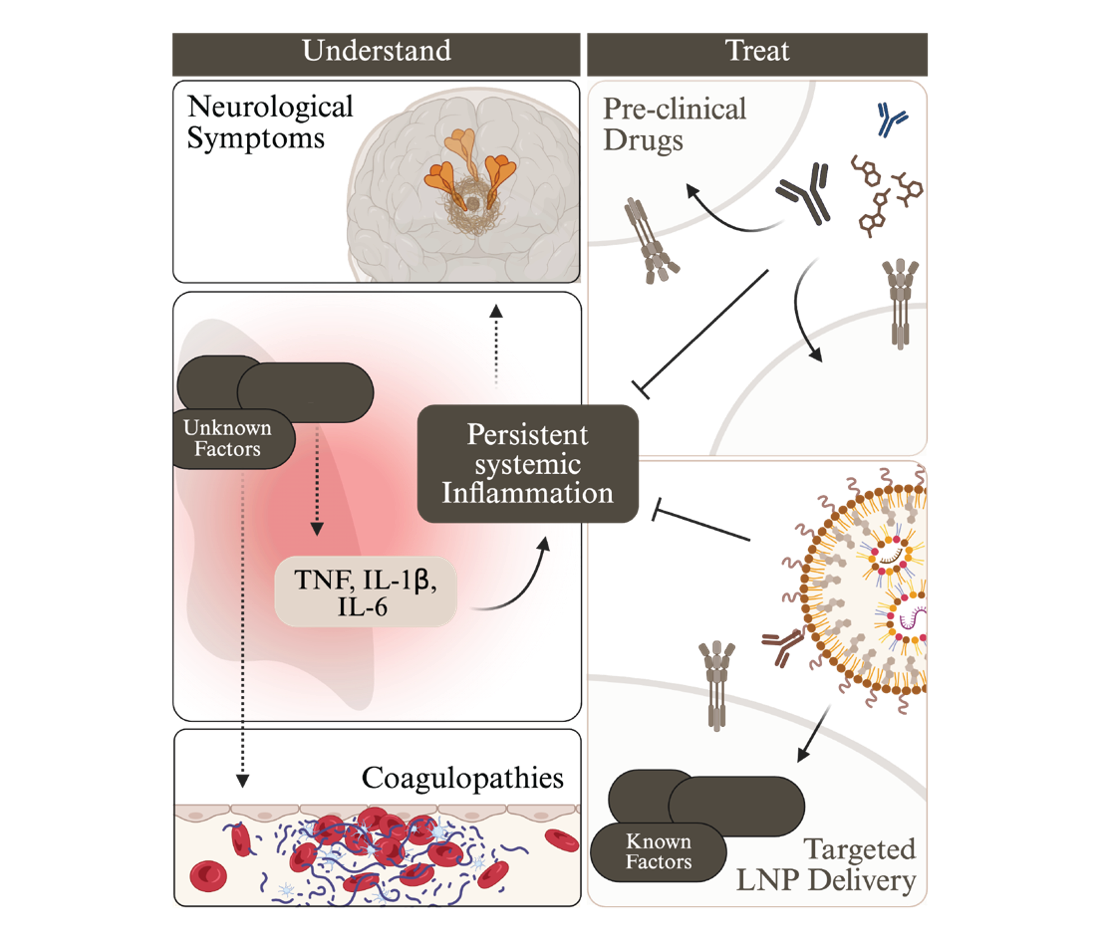
Figure 3: Overview of our objectives.
Complete publication list: https://orcid.org/0000–0002-7901–9833
- S. Bader*, …, M. Pellegrini* and M. Doerflinger*, “Non-apoptotic caspase‑8 is critical for orchestrating exaggerated inflammation during severe SARS-CoV‑2 infection.“ Nature Comms., 2025. *Corresponding
- M. Bader#, D. Calleja#, S. Devine S#, N. W. Kuchel# …, D. Komander, “A novel PLpro inhibitor improves outcomes in a pre-clinical model of long COVID”, Nature Comms., 2025
- M. Bader, …, M. Pellegrini and M. Doerflinger, “IL-1b drives SARS-CoV‑2 disease, independently of the inflammasome and pyroptotic signalling”, Cell Death Differ., 2025
- M. Bader, …, M. Doerflinger, “Necroptosis does not drive disease pathogenesis in a mouse infective model of SARS-CoV‑2 in vivo”, Cell Death Dis., 2024
- M. Bader#, J. P. Cooney# …, M. Pellegrini, “SARS-CoV‑2 mouse adaptation selects virulence mutations that cause TNF driven age-dependent severe disease with human correlates”, PNAS, 2023
- M. Bader, …, A. L. Samson, “Endothelial Caspase‑8 prevents fatal necroptotic hemorrhage caused by commensal bacteria” Cell Death Differ., 2022
- M. Bader, …, M. Doerflinger; “Programmed cell death: the pathways to severe COVID-19?”, Biochem J., 2022
- P. Cooney, …, S.M. Bader, …, M. Pellegrini, “Combination antiretroviral therapy and MCL‑1 inhibition mitigate HTLV‑1 infection in vivo” Cell, 2025.
- P Preston, …, S. M. Bader, …, Marc Pellegrini, “A necroptosis-independent function of RIPK3 promotes immune dysfunction and prevents control of chronic LCMV infection”, Cell Death Dis., 2022.
- P Preston, …, S. M. Bader, …, Marc Pellegrini, “Epigenetic Silencing of RIPK3 in Hepatocytes Prevents MLKL-mediated Necroptosis from Contributing to Liver Pathologies”, Gastroenterology, 2022
- Renz, A.,… M. Bader, … Michael Schindler and Christoph Kaleta. “Metabolic Modeling Elucidates Phenformin and Atpenin A5 as Broad-Spectrum Antiviral Drugs”, Preprints, 2022.
- D. Stutz, …, S. M. Bader, …, M. Pellegrini, “Macrophage and neutrophil death programs differentially confer resistance to tuberculosis”, Immunity, 2021.
- Doerflinger, …, S. M. Bader, …, M. J. Herold, “Flexible usage and interconnectivity of diverse cell death pathways protect against intracellular infection,” Immunity, 2020.
